A functional systems approach to understanding verbal-motor integration in individuals with Down syndrome
In this paper we present the background, development and application of a functional systems approach to understanding verbal-motor integration characteristic of persons with Down syndrome. Based on our initial work utilising noninvasive, neuropsychological procedures, we have forwarded a specific model of brain-behavior relations in persons with Down syndrome. The crucial characteristic of the model is the proposed functional disconnection of brain areas responsible for speech perception and movement organization. In addition to describing the model, we summarize our recent work designed to test, refine, and extend it.
Chua, R, Weeks, D, and Elliott, D. (1996) A functional systems approach to understanding verbal-motor integration in individuals with Down syndrome. Down Syndrome Research and Practice, 4(1), 25-36. doi:10.3104/reviews.60
Introduction
Over the last ten years our laboratories at McMaster University, and more recently, Simon Fraser University and the University of Alberta, have been actively engaged in research concerned with cerebral organization in adults and adolescents with Down syndrome. Our goal has been to determine how patterns of brain organization unique to Down syndrome may contribute to both the specific and general information processing capabilities of individuals with Down syndrome.
Much of what we know about brain-behavior relations in general comes from clinical studies involving people who have suffered a stroke or a head injury which has damaged a localized area of the cerebral cortex. Since the late 1800s, it has been recognized that in most right-handed people the left cerebral hemisphere plays a special role in speech and language. This is because left hemisphere brain damage is much more likely to result in expressive (Broca, 1865) and receptive (Wernicke, 1874) speech and language problems (aphasia) than comparable right hemisphere damage. The left hemisphere also appears to be specialized for the organization and control of voluntary movement, including speech movements. Thus, left hemisphere damage is more likely to result in motor learning and motor control problems (i.e., apraxia; see Roy, 1985 for a review).
Although left hemisphere specialization for speech, language and motor control was apparent to clinicians over 100 years ago, the left hemisphere is no longer referred to as the "dominant" hemisphere by most neuropsychologists. Studies examining persons with right hemisphere brain damage indicate that these individuals are more likely to suffer deficits in tasks that require the perception of the spatial relations between objects in the environment (Jackson, 1958), object recognition (De Renzi, 1968) and selective attention (Heilman and Watson, 1977). It also appears that the right cerebral hemisphere may be involved in the perception and expression of emotion (Ley and Bryden, 1979). Thus, the right hemisphere also appears to regulate a number of important functions.
In addition to the clinical investigations involving brain-injured people, experimental neuropsychologists have developed a number of noninvasive techniques for examining brain-behavior relations in the intact brain. Our initial interest in Down syndrome developed as a result of several studies that employed a procedure referred to as dichotic listening.
The dichotic listening paradigm is a noninvasive method for examining cerebral specialization for speech perception. Typically, participants are presented with pairs of letters, digits, or words simultaneously to the right and left ears through headphones. In what is termed a "free recall" situation, the participants are asked to report every sound they hear. Alternatively, a "selective listening" procedure may be employed in which the participant is asked to report sounds from one or the other ear. Regardless of the procedure, most right-handed children and adults correctly report more right ear items than left ear items. Because of the contralateral or crossed nature of the major auditory pathways, this right ear advantage for the perception of speech sounds has been taken to reflect left hemisphere specialization for speech and auditory language function.
The majority of dichotic listening studies involving children and adults with Down syndrome have reported quite different results. Specifically, persons with Down syndrome usually display an atypical (i.e., reversed) left ear/right hemisphere advantage for the perception of speech sounds (Bowler et al., 1985; Elliott and Weeks, 1993; Hartley, 1981; Pipe, 1983; Zekulin-Hartley, 1981, 1982; cf. Tannock et al., 1984) regardless of the type of dichotic listening procedure employed (Giencke and Lewandowski, 1989; see Elliott et al., 1994 for a review). In a recent meta-analysis involving all the published dichotic listening studies conducted with persons Down syndrome, we found that (a) relative to other people with mental disabilities, (b) relative to people without disabilities, and (c) relative to a theoretical laterality index of zero, children and adults with Down syndrome display a reliable left ear (right hemisphere) advantage for the perception of speech sounds (Elliott et al., 1994).
These dichotic listening findings suggest that the trisomy 21 karyotype may carry with it a distinct pattern of cerebral organization. Hartley (1982, 1986) and Pipe (1988) have speculated that this atypical brain organization may be responsible for some of the specific information processing problems experienced by children and adults with Down syndrome. For example, sequential language problems (e.g., Ashman, 1982; Hartley, 1982) may result from individuals with Down syndrome relying upon right hemisphere information processing systems that are not optimally organized for that type of task. The right hemisphere is often characterized as a more parallel processor of information (e.g., Semmes, 1968) and thus better equipped for more holistic types of task such as space perception, but not sequential types of tasks such as the perception and production of language.
Our Research Programme
Toward A Neurobehavioral Model
Intrigued by the findings of reversed cerebral specialization for speech perception, but also influenced by our own backgrounds in kinesiology and human motor control, our initial studies focused on cerebral specialization for the organization and control of voluntary limb movements. Approximately 90% of the general population is right-handed (Bryden et al., 1996). This characteristic is thought to be the result of the fact that the distal musculature of the right hand is almost exclusively controlled by the left cerebral hemisphere (i.e., contralateral neural pathways) which seems to play a special role in the organization of movement for both sides of the body. That is, for most of us, the right hand has direct access to the neural system that is most efficient at selecting and timing the muscular forces that move the limbs (Elliott and Chua, 1996). Right hand preference also appears to be the norm for the majority of persons with Down syndrome, with estimates ranging from 75-85% (e.g., Batheja and McManus, 1985; Elliott et al., 1994; Murphy, 1962; Pickersgill and Pank, 1970). Our approach to studying manual and presumably cerebral asymmetries in persons with and without Down syndrome was to examine performance differences between simple tasks, since preference can be influenced by a great number of social variables (Harris, 1990).
In two initial studies, we had participants with and without Down syndrome finger tap as rapidly as possible with the index finger of the right and left hands (Elliott, 1985; Elliott et al., 1986). Finger-tapping was chosen as a task because rapid and consistent performance depends on the ability of the contralateral cerebral hemisphere to precisely coordinate muscular activity (i.e., the specification and timing of muscular forces). Interestingly, we found that our participants with Down syndrome exhibited the same pattern of performance as participants without Down syndrome. That is, they were faster and, more importantly, more consistent in the timing of their individual finger taps when tapping with their right hand. Given the explanation for right hand superiority, this type of finding indicates that, like most other individuals, people with Down syndrome are left hemisphere specialized for motor control.
We proceeded to perform two further studies on cerebral specialization for motor control using a transfer of learning paradigm that we again borrowed from the experimental neuropsychology literature. This procedure is based on the finding that intermanual transfer of training is asymmetric. Specifically, when an individual practices a new motor task such as rapidly producing a specific sequence of key presses with one hand, there is a certain degree of transfer of training to the other hand. In this situation however, the pattern of transfer of learning is asymmetric, with more transfer of training from the left hand to the right hand than the reverse (Hicks, 1974; Taylor and Heilman, 1980). Taylor and Heilman (1980) have suggested that this asymmetry in transfer of training is due to left hemisphere specialization for motor control. The notion is that when the left hand is actively practicing the motor task both cerebral hemispheres must be involved - the right hemisphere because it controls the distal musculature for the left hand, but also the left hemisphere because of its specialized role in movement organization. This situation leads to greater transfer of training than when the right hand is active since in this latter situation only the left hemisphere is required to be active. For our purposes this experimental approach seemed ideal for examining cerebral specialization for motor control in adults with Down syndrome.
In our experiments we had individuals with and without Down syndrome learn a rapid finger sequencing task with either the right or the left hand. After practice, we examined how much of that training had transferred to the unpracticed hand. Both groups of participants exhibited more transfer of training if they were trained with the left hand. Given Taylor and Heilman 's (1980) explanation of asymmetric transfer, this finding again suggests that persons with Down syndrome are left hemisphere specialized for the organization and control of movement.
The neural systems that are responsible for the organization and control of movement are thought by some investigators (e.g., Kimura, 1979) to be the same systems that underlie left hemisphere specialization for speech production and perhaps expressive language function in general. That is, the left cerebral hemisphere appears to be responsible for the precise motor control necessary for the complex movement transitions in gestural and spoken language. This of course creates a bit of a paradox for persons with Down syndrome, who appear to be right hemisphere specialized for speech perception, but left hemisphere specialized for the organization and control of movement. Our next challenge then was to attempt to examine cerebral specialization for speech production in persons with Down syndrome. Once again, we borrowed an experimental paradigm from the neuropsychological literature.
In this study, we (Elliott et al., 1987) asked young adults with and without Down syndrome to finger-tap as rapidly as possible with the right and left index fingers. In one situation they performed the finger-tapping task while also speaking aloud. This simply involved participants repeating a series of high frequency words that they heard through headphones. Typically, for most right-handed people the concurrent speech interferes with right hand, but not left hand, finger-tapping performance (Kinsbourne and Hicks, 1978). This pattern of performance is thought to occur because the neural structures responsible for right hand motor control and speech production both reside in the left cerebral hemisphere, creating within-hemisphere interference. When the left hand is tapping, the areas of the brain responsible for left hand performance (i.e., right hemisphere) and speech production (i.e., left hemisphere) are functionally distant (Kinsbourne and Cook, 1971; Kinsbourne and Hicks, 1978). Our goal of course was to gain some understanding of cerebral specialization for speech production by examining the pattern of interference for our participants with Down syndrome. Because, like most right-handed individuals, right-handers with Down syndrome exhibited greater dual task interference when performing with the right hand, it appeared that as a group they are also left hemisphere specialized for speech production (Elliott et al., 1987; see also Piccirilli et al., 1991). While this finding is consistent with findings indicating left hemisphere specialization for motor control, it is extremely interesting in view of the dichotic listening findings indicating that persons with Down syndrome appear to be right hemisphere specialized for speech perception.
A Model of Functional Cerebral Organization for Down syndrome
Based on the findings that young adults with Down syndrome exhibit anomalous right hemisphere specialization for speech perception, but that the left hemisphere appears to play a special role in the organization and control of movement, including speech movements, we developed a specific neurobehavioral model to guide our research (Elliott and Weeks, 1993; Elliott et al., 1987b; Elliott et al., 1994; Weeks and Elliott, 1992). The main feature of our model is the apparent dissociation or disconnection of speech perception (right hemisphere) and movement production, including the production of speech movements (left hemisphere) in persons with Down syndrome. Drawing on a number of cognitive and psychometric studies which indicate that persons with Down syndrome have particular difficulty performing a variety of tasks that require both the perception of speech sounds and the production of complex oral or manual movements (e.g., Ashman, 1982; Hartley, 1986; Mahoney et al., 1981), we suggested that there is a cost associated with this particular pattern of brain organization. In summary, we proposed that the "separation of perception and movement production systems leads to a breakdown in communication, presumably because interhemispheric transmission between these systems results in the partial loss of information" (Elliott and Weeks, 1993, p. 104). Our model is illustrated in Figure 1.
General Model
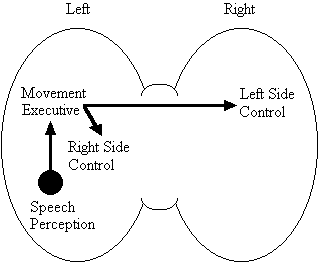
Down Syndrome Model
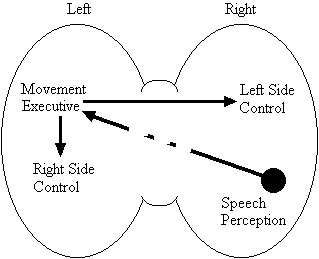
Figure 1. A model of functional cerebral organization in persons with Down syndrome.
It is one thing to fit existing evidence to a model of brain-behavior relations. It is quite another to conduct experiments that are designed specifically to test particular aspects of a model. One prediction of our model is that persons with Down syndrome will have difficulty, relative to persons of a similar mental age, performing oral or manual movements on the basis of verbal direction. They should have no such difficulty if the directions are provided visually. Adapting a battery developed by other investigators (De Renzi et al., 1966; Kools et al., 1971) to examine a set of movement disorders termed apraxia, we set out to test this explicit prediction.
Evaluation of the model
In two different studies (Elliott and Weeks, 1990; Elliott et al., 1990), we had adults of a similar mental age, with and without Down syndrome, perform individual movements or movement sequences on the basis of verbal instruction or following a demonstration. The manual movements included actions such as "clap your hands" and "put your finger in your ear", while the oral movements were actions such as "buzz like a bee" and "blow out a match". While the participants with Down syndrome performed as well as and sometimes better than the other participants following a demonstration, they had greater difficulty performing the same movements on the basis of verbal instruction. Moreover, the difference in their verbal versus demonstration performance became more pronounced as the length of the movement sequence was increased. This problem with the verbal-motor condition did not appear to be due to speech comprehension or memory for the verbal instructions, because when participants with Down syndrome were asked to point to pictures of a research assistant performing the same movements, they performed as well as the control group. As our model predicts, the problem appears to be in translating the verbal instruction into the appropriate sequence of movements.
Although it is tempting, when asked about the practical implications of our findings, to state that persons with Down syndrome should be taught novel motor tasks with a great deal of visual demonstration and very little verbal instruction, these inferences about motor learning based on performance findings do not necessarily hold true in all situations. In the motor learning literature, there are many examples of instructional techniques and/or schedules that benefit performance in the short-term while actually proving to be detrimental to long term retention (see Salmoni et al., 1984 for a review). With this in mind, we (Elliott et al., 1991) decided to conduct a study in which we taught groups of participants with and without Down syndrome a novel motor task using a verbal instructional protocol.
A group of adults with Down syndrome as well as control groups with both a similar chronological age and a similar mental age were taught a novel movement sequencing task. The task involved moving the preferred hand from a start position to a lever that they had to shift to the right, a headlamp switch that they had to pull and a dial that they had to turn to the left. Before each trial the participants were verbally told the activities they were to perform and the order of these activities. Following an auditory signal they were to complete the sequence of movements as rapidly as possible in the appropriate order. Although participants in all three groups improved at the task with practice, the participants with Down syndrome had more difficulty with the task than participants in the other two groups during a retention test when the verbal cues were withdrawn. They were particularly slow at initiating the movement sequence suggesting that they had difficulty internalizing the verbal instructions that were available during acquisition, but withdrawn for the retention test. While we need to conduct a similar study in which the instructional mode is visual, this study at least suggests that the verbal-motor problems experienced by persons with Down syndrome in tests of motor performance generalize to motor learning.
In a recent study, one of our students (Le Clair and Elliott, 1995) attempted to identify the locus in the information processing chain of events that gives rise to the verbal-motor difficulties that appear to affect both motor performance and learning. Adult participants with and without Down syndrome attempted to initiate and complete one of two target-aiming movements as rapidly as possible when a visual signal identified the specific movement to be made. In a control condition the two movements were equally probable (i.e., p = .50). In another condition participants were given either visual or verbal advance information about which movement was likely to be required. The advance information was reliable 80% of the time. On 20% of the trials it was invalid and the unexpected movement was required. While all participants were able to benefit from the advance information when it was presented visually (i.e., they were faster at initiating and completing their movements), the participants with Down syndrome did not benefit to the same extent as persons without Down syndrome when the advance information was presented verbally. Because they were slower at initiating their movements in the 80% verbal condition, it appeared that participants with Down syndrome have difficulty preparing a specific movement on the basis of verbal instruction. Paradoxically, on the trials in which the verbal information was invalid (p = .20), the participants with Down syndrome were just as disrupted as the other participants (i.e., slower at initiating and completing the movement than in the control condition). Thus, it appeared that while individuals with Down syndrome attempt to employ the verbal advance information to improve their performance, they are unsuccessful in doing so.
In the studies we have discussed thus far, we adopted a group differences approach in which we compared a group of participants with Down syndrome to other individuals of a similar mental and/or chronological age. Certainly, in any cognitive or perceptual-motor task there is at least as much variability within a group of persons with Down syndrome as there is variability between groups. Because our model is based on a dissociation between speech perception and movement organization, it follows that individuals with Down syndrome who display the greatest degree of functional separation between these two subsystems should also exhibit the most pronounced difficulties on tasks that require movement organization on the basis of perceived speech. Therefore, in a subsequent test of our model we (Elliott and Weeks, 1993) used a dichotic listening procedure to obtain an index of cerebral specialization for speech perception (i.e., a laterality index) and then attempted to determine if the degree of right hemisphere advantage was related to verbal-motor processing performance.
As in previous studies we were able to demonstrate that participants with Down syndrome exhibited a left ear/right hemisphere advantage for the perception of, in this case, pairs of digits. On a variation of the apraxia battery discussed earlier, our participants with Down syndrome again had difficulty in performing one, two and three element movement sequences on the basis of verbal instruction, but not demonstration. From an individual differences perspective, the important finding in this study was a moderate but statistically significant relation between the dichotic listening laterality index and the verbal portion of the apraxia battery. Specifically, those individuals with Down syndrome exhibiting a greater degree of right hemisphere specialization for speech perception tended to do more poorly in organizing movements on the basis of verbal instruction. There was no apparent relation between the dichotic scores and the apraxia battery for people without Down syndrome. Thus, once again we have some modest support for our model.
In addition to the dichotic listening test and the apraxia battery, we (Elliott and Weeks, 1993) also had participants complete a series of tests taken from the Raven's Coloured Progressive Matrices (Raven, 1965). This test involves visual pattern discrimination and has been suggested to tap what is typically regarded as right hemisphere visual-spatial function (see Costa, 1976 and Denes et al., 1978 for a discussion of right and left hemisphere involvement). An interesting finding in this study was that the persons with Down syndrome who had the most pronounced right hemisphere dichotic advantages performed poorly on the Raven's. This suggests that there may be a cost for the development of right hemisphere language function; that is, the more typical right hemisphere visual-spatial function may suffer.
In summary, while persons with Down syndrome appear to be right hemisphere specialized for the perception of speech sounds, they show the same pattern of cerebral specialization as the general population for the organization and control of movement, including speech movements. This functional separation of two systems that are usually subserved by the same cerebral hemisphere (i.e., the left hemisphere) appears to lead to difficulty in performing tasks that involve the intimate interaction of the two systems. Presumably, there is a loss of information due to interhemispheric communication. Moreover, the development of right hemisphere receptive language in persons with Down syndrome may influence more than just verbal-motor behavior. Specifically, it may have an impact on visual-spatial processing normally subserved by the right cerebral hemisphere.
Refinement and Extension of the Model
In several recent studies we have attempted to extend our understanding of brain-behavior relations in persons with Down syndrome to right hemisphere spatial function, and to language function in more than just the auditory modality. The examination of spatial function was initially motivated by the relation we observed between dichotic listening scores and performance on the Raven's Coloured Progressive Matrices (Elliott and Weeks, 1993).
Spatial processing in individuals with Down syndrome
As discussed earlier, most people, including individuals with Down syndrome, perform tasks that require the precise timing of muscular forces (e.g., finger-tapping, finger-sequencing) better with their right hand than their left hand. However, there are manual tasks that right-handed people can perform better with their left hand. For example, people typically can make spatial judgments of orientation (Benton et al., 1978), match nonsense shapes (Witelson, 1974) and reproduce spatial positions (Roy and MacKenzie, 1978; see also Carnahan and Elliott, 1987) better with the left hand than the right hand. Presumably this left hand advantage is related to a right hemisphere proficiency at more holistic/spatial processing (i.e., crossed sensory and motor pathways).
In a recent study, we (Elliott et al., 1995) attempted to examine cerebral specialization for spatial processing in adults with Down syndrome by having them perform a bimanual tactile matching task. Previous work has shown this task to yield a left hand/right hemisphere advantage (e.g., Witelson, 1974). Participants with and without Down syndrome were presented with a pair of rubber shapes and were required to simultaneously manipulate these shapes without visual feedback available, afterwards matching the pair from a display consisting of 6 shapes. Results revealed similar patterns of asymmetry between the participants with Down syndrome and the control participants. In particular, left handed participants with Down syndrome exhibited a significant left hand advantage for the task.
In a second experiment (Elliott et al., 1995), we examined asymmetries in visuospatial processing. Previous work using visual field presentations have revealed that presentation of spatial stimuli to the left visual field leads to more effective processing than presentation to the right visual field (e.g., Kimura, 1966; Umilta et al., 1974 ). This visual field asymmetry is thought to reflect the differential processing capability of the contralateral hemisphere that has initial access to the information. There has been little work done with populations with mental disabilities using this methodology due to a requirement of the visual field protocol in which participants must fixate a central position prior to stimulus presentation. Persons with mental disabilities have difficulty following fixation instructions. We attempted to circumvent this problem by adapting a method developed by Smith and colleagues (1986).
Using their two index fingers, our participants moved a mouse on a graphics tablet in order to displace a cursor presented on a computer monitor onto a small target located centrally on the monitor. At the moment the cursor entered the target, the stimulus was presented. Thus, the target served as a fixation point, and we assumed that the participant had to maintain fixation of the target in order to accurately center the cursor. This method was used to examine visual asymmetries in a dot enumeration task. Participants were briefly presented a set of 2 - 6 dots, randomly arranged within a circular space, either to the left or right of the fixation target. Participants reported the number of dots they saw. We found that both participants with and without Down syndrome displayed a left field advantage in this task (Elliott et al., 1995). Once again, this type of asymmetry is taken to reflect right hemisphere superiority for processing spatial relations.
Thus, for both the tactile matching task and the visual field dot enumeration task, participants with Down syndrome displayed similar patterns of asymmetry compared to age matched control participants. This suggests that, like people without mental disabilities, most persons with Down syndrome are right hemisphere specialized for the processing of spatial information. We find little evidence to suggest that persons with Down syndrome exhibit syndrome specific peculiarities with respect to cerebral lateralization for spatial function. Consequently, if both receptive speech and visual-spatial processing are subserved by the right hemisphere in individuals with Down syndrome, it is the former type of processing that is most compromised. Thus, contrary to the general "reversed cerebral specialization model" proposed by Hartley (1981, 1982) our work, in concert with the dichotic listening studies, has supported the position that atypical cerebral organization of function in persons with Down syndrome is confined to speech perception.
Language processing in individuals with Down syndrome
Although we have obtained support for our model, the expression of the functional dissociation has been limited to the auditory perception of linguistic material. Therefore, in a second pair of experiments, we (Weeks et al., 1995) extended our investigations of speech perception and examined whether the atypical specialization for receptive language in persons with Down syndrome is limited to the auditory modality, or can also be extended to haptic and visual perception. We used similar methods as in our examinations of spatial processing.
As we have discussed, many of the findings on which our model is based have been obtained from studies employing dichotic listening. Therefore we wanted to employ tactile and visual analogues of the dichotic listening task. Witelson (1974) has provided a task that can serve as a tactile equivalent to dichotic listening. In separate experiments participants were either required to feel pairs of nonsense forms or letters and later select (nonsense form) or recall (letters) items that had been felt. A left-hand/right hemisphere advantage was observed for the nonsense forms task and a right-hand/left-hemisphere advantage was obtained for the letters task (Witelson, 1974). Similarly, Gibson and Bryden (1982) used shapes and letters cut from sandpaper that were moved slowly across the participant's fingertips. Stimuli were presented in pairs and participants were cued as to which stimulus to report first. Consistent with Witelson's (1974) findings, letter identification was superior with the right hand whereas shape identification was superior with the left hand. The implication of these data is that participants with left hemisphere specialization for receptive language will demonstrate a right-hand advantage for tactually presented linguistic material (Cioffi and Kandel, 1979; Varga-Khadem, 1982).
We employed this tactile methodology to examine cerebral specialization for receptive language in the tactile modality in individuals with Down syndrome (Weeks et al., 1995). Our interest was to determine whether individuals with Down syndrome would exhibit a right-hand advantage or a reversed advantage as they do for dichotic listening. We presented participants with pairs of shapes that corresponded to letters. Participants simultaneously manipulated the letter shapes and attempted to identify the corresponding pair of letters from a display of 6 letters. Our participants with Down syndrome exhibited a left hand advantage on this task. The control group did not manifest any manual asymmetries.
Although one might initially expect a right hand advantage for this task (e.g., Witelson, 1974), our observation of a left hand advantage lends itself to an interpretation consistent with the features of our model. Specifically, although the stimuli are linguistic symbols, they are also spatial in nature. Participants may therefore employ a system in which the letter stimuli first undergo spatial processing prior to linguistic processing. The former analysis is more efficiently performed by the right hemisphere and the latter by the left hemisphere. However, because both linguistic and spatial processing are presumed to be subserved by the right hemisphere in persons with Down syndrome, the link between these two functions facilitates processing of the tactile letters, and is expressed as a left hand advantage in this task.
In a second experiment (Weeks et al., 1995), we employed the visual field and fixation protocol we described above (Elliott et al., 1995), presenting letters as stimuli, to serve as the visual analogue for dichotic listening. Once participants had centered the cursor on the target, 3 letters, arranged in a vertical column, were presented to either visual field, and participants attempted to identify these letters. We provided a chart of the possible letters to aid participants with Down syndrome with their verbal report. We obtained only a slight left field/right hemisphere advantage for the group of participants with Down syndrome. Despite the lack of a field advantage for the control group, the direction of results for persons with Down syndrome were as expected, taking into account the results of the tactile experiment and the predictions of our model.
These two experiments (Weeks et al., 1995) on haptic and visual language processing suggest that the atypical left ear/right hemisphere advantage for the perception of speech sounds (Elliott and Weeks, 1993) may also generalize to the haptic and visual perception of linguistic information. Overall these data lend themselves to an interpretation consistent with the pattern of lateralization predicted by our model. That is, the left hand advantage for haptic processing and the left field advantage for visual processing of linguistic material suggest that, at least for this relatively homogeneous group of verbally fluent participants (Rondal, 1994), language perception is mediated by the right hemisphere. Further, these experiments complement our previous results on spatial processing (Elliott et al., 1995) by providing further evidence to support the selective nature of the atypical cerebral organization in persons with Down syndrome.
New Directions
In our work to date, we have employed neuropsychological techniques to study the nature of perceptual-motor behavior in persons with Down syndrome. This approach has offered us a window into the nature of cerebral organization and brain-behavior relations in persons with Down syndrome and allowed us to formulate the basic tenets of a model. We believe that this model can provide a rich source of research questions for a next round of inquiry that could raise our level of understanding regarding the complex nature of perceptual-motor integration in Down syndrome.
We have begun two new research directions to further examine the implications of our model. One direction is to determine if the disconnection between functional systems predicted by our model impacts upon dynamic, coordinative actions requiring visual-motor or auditory-motor integration. The second direction involves the use of electrophysiological measures to examine the active cortical systems in the brain that underlie the performance of verbal and motor tasks by persons with Down syndrome. By extending our observations beyond the behavioral level to the level of the neural systems, the internal and external validity of our model could be strengthened by obtaining more "direct" evidence.
Coordinative Actions
As we have outlined in the preceding sections, one important prediction of our model for which support has been found, is that persons with Down syndrome will exhibit specific difficulty on tasks which require the cooperation of the functional systems responsible for speech perception and movement organization (Elliott et al., 1990). This difficulty may also be characterized as a problem in integrating perception and action (i.e., speech perception and movement production). This characterization allows us to employ novel tools and principles from the domain of coordination dynamics to further investigate whether this prediction from our model is limited to discrete limb and oral movements (see Elliott and Weeks, 1993, for a review) or extends to coordinative actions requiring visual-motor or auditory (verbal)-motor coordination.
The dynamical systems approach in motor behavior has made considerable progress in the study of movement coordination (see Turvey, 1990 for a review). A number of investigators have successfully employed this paradigm to study the coordination of perception and action, namely, visual-motor coordination (Byblow et al., 1995; Schmidt et al., 1990; Wimmers et al., 1992), and auditory-motor coordination (Kelso et al., 1990). In light of the predictions from our disconnection model, we have been interested in determining whether persons with Down syndrome have greater difficulty in coordinating action with auditory information (e.g., speech) compared with visual information. For example, consider a movement task in which participants are required to coordinate rhythmic, oscillatory movements with either a visual or auditory signal. If our model extends to the domain of perception-action coordination, we would expect that persons with Down syndrome will achieve better coordination when the movement requires the cooperation of the visual perception and movement systems compared to when the movement requires the cooperation of the auditory (speech) perception and movement systems. Moreover, if the nature of perception-action coordination in persons with Down syndrome can be so characterized, it remains to be determined whether the verbal-motor difficulties experienced by these persons are specific only to speech perception or extend to auditory perception in general.
As a starting point, we have been examining auditory-motor and visual-motor coordination in persons with Down syndrome. Our interest is in determining the consistency of movement coordination with either a visual or auditory stimulus. Our task requires participants to perform rhythmic forearm movements with a lever, which moves in a left-right dimension about its axis, in synchrony with a computer-generated visual stimulus and an auditory stimulus. Five participants with Down syndrome participated in our initial study. Participants sat with their midlines aligned with a computer monitor placed at eye level and grasped a lever located directly in front of their midline. The visual stimulus was a computer-generated cursor which flashed briefly between left and right positions on the monitor. The auditory stimulus was a 1000 Hz tone of 100 ms duration and was provided coincident with each occurrence of the visual stimulus. The stimuli completed a left-right cycle once every 2 seconds.
At the start of a trial, the visual stimulus cycled discretely back and forth on the monitor. The appearance of the visual stimulus at each of its left and right positions was coincident with the presentation of the auditory stimulus. Participants were then asked (through verbal directions and visual demonstrations) to synchronize their movements such that when the visual stimulus was on the left side, their movement should also be at its left endpoint, and that when the visual stimulus was on the right side, their movement should also be at its right endpoint. They were also advised of the auditory stimulus, which would be coincident with the visual stimulus.
Once a participant was able to establish coordination with the stimuli, the trial proceeded. There were two types of trials, comprising two transfer conditions. In the visual transfer condition, the visual-auditory stimuli were presented for 15 cycles for the first half of the trial, after which the auditory stimulus was removed, and participants were required to maintain coordination with the remaining visual stimulus on the second half of the trial. In the auditory transfer condition, the visual-auditory stimulus combination was presented for 15 cycles for the first phase of the trial, after which the visual stimulus was removed and participants were required to maintain coordination with the auditory stimulus alone on the second phase. We collected kinematic data from movement of the lever and calculated the coordinative relation between the participant's movement and the visual / auditory stimuli (for specific details regarding the type of data collection and data reduction procedures we employed, see Byblow et al., 1995).
We examined the relative phase between the participant's movement and the stimuli. The relative phase provides a measure of the position-time relation (the coordination) between the stimuli and movement. For example, if a participant's movements were in perfect spatial and temporal synchrony with the visual stimulus, the coordinative relation can be regarded as in-phase, associated with a relative phase value of 0 degrees. If a participant's movements were in perfect temporal, but opposite spatial, synchrony with the visual stimulus (e.g., movement at left endpoint - stimulus at right endpoint), the relation can be regarded as out-of-phase, associated with a value of 180 degrees. The relative phase can assume values between 0 and 360 degrees, ranging from perfect coordinative relations to those relations in between. With respect to coordination with the auditory stimulus, because the tone was present at both endpoints of the cycle with no differentiation spatially, in-phase and anti-phase coordination were essentially equivalent. Although there are many interesting issues regarding in-phase versus out-of-phase coordination, of primary interest to us at this stage was the consistency in a participant's coordination as a function of the stimulus conditions, and the amount of time in which participants were outside of either in-phase or out-of-phase synchrony with the stimuli.
The uniformity of the relative phase relations provides information about the consistency and stability of coordination (see Byblow et al., 1995, for specific calculations). Individual-subject analyzes were performed on each data set from a participant, using a 2 transfer conditions (visual transfer, auditory transfer) x 2 trial phase (first phase, second phase) mixed analysis of variance. The transfer conditions differed with respect to the second phase (2nd half) of the trials only. Thus if there were differences between conditions in which the visual stimulus is presented alone or the auditory stimulus is presented alone, we would generally expect an interaction between transfer condition and trial phase. Significant effects were obtained only for 2 of 5 participants. In one participant, the consistency of coordination was found to be greater overall for the auditory transfer trial than for the visual transfer trial, F(1,28) = 20.97, p < .001, and for the first trial phase (both stimulus sources) than for the second trial phase (single stimulus source), F(1,28) = 22.07, p < .001. Further, the primary source of the differences was attributable to the second phase of the trials, during which coordination with the auditory stimulus was more consistent than with the visual stimulus, F(1,28) = 28.38, p < .001. In a second participant, there was again greater consistency overall for the auditory transfer than the visual transfer trials, F(1,14) = 6.76, p < .025, with the difference tending to arise primarily during the second trial phase, F(1,14) = 4.05, p < .065, when only a single stimulus source was available.
For the same two participants above, significant effects were also found for the proportion of time during which coordination was outside of an in-phase region or out-of-phase region (see Byblow et al., 1995, for specific calculations). If one is able to maintain coordinative relations within a region about in-phase or out-of-phase throughout a trial, then there should be little time spent in intermediate coordinative relations outside these regions. An increase in the amount of time outside these two regions is taken to indicate poor coordinative synchrony in a participant's movements with the stimulus. In the first participant (same as above), less time was spent overall in intermediate coordinative relations during auditory transfer trials than during visual transfer trials, F(1,28) = 10.71, p < .005, and during the first phase of trials than the second phase, F(1,28) = 29.08, p < .001. Again, the difference was primarily attributable to the second phase of the trial, during which time only a single stimulus source was available, F(1,28) = 49.55, p < .001. Coordination with the auditory stimulus was maintained better than with the visual stimulus. In the second participant, coordination during the auditory transfer trials was, on average, maintained within in-phase or out-of-phase regions to greater extent than during visual transfer trials, F(1,14) = 6.87, p < .025. Once again, the effect of transfer condition was primarily seen during the second phase of the trial, F(1,14) = 4.80, p < .05. Examples of time series taken from a participant are shown in Figure 2. Illustrated in panels A and B are a visual transfer trial in which the participant's coordination was more consistent during the first phase (visual + auditory stimuli) than the second phase (visual stimulus), and an auditory transfer trial in which coordination was essentially maintained throughout the trial, respectively.
A
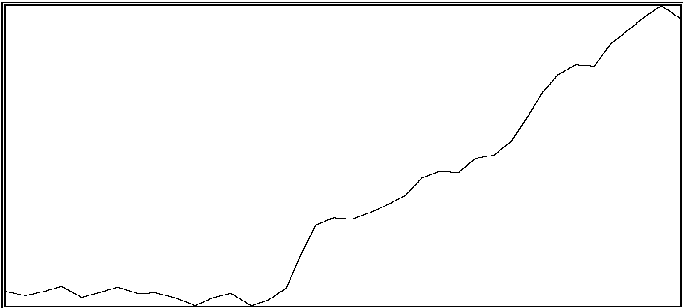
B
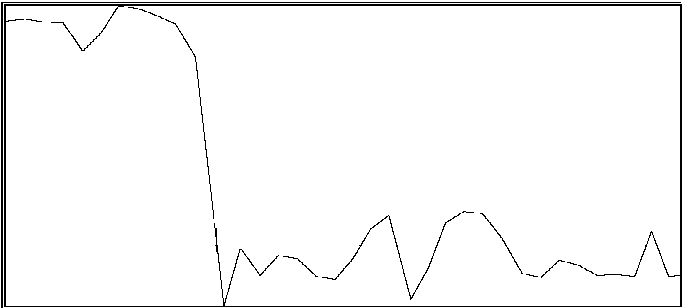
Figure 2A and 2B. Time series of coordinative relation, relative phase, for a visual transfer trial (A) and auditory transfer trial (B).
Panel A illustrates loss of coordination during the second phase of the trial. Upper and lower dashed lines demarcate 180 degrees and 0 degrees, respectively. Panel B illustrates a momentary loss of coordination which is then reestablished. Upper and lower dashed lines demarcate 0 degrees and 180 degrees respectively.
In summary, our initial work examining perception-action coordination in individuals with Down syndrome has yielded results somewhat contrary to our expectations. The two participants that exhibited differences in coordination as a function of stimulus conditions showed better coordination overall when an auditory stimulus was present. It is possible that this stimulus source was more salient and therefore served as a better source for movement synchronization. Thus, in the case of these two participants, auditory-motor coordination proved to be more consistent than visual-motor coordination. As the predictions of our model specifically relate to verbal-motor versus visual-motor coordination, we are presently designing a verbal-motor condition in which the stimulus source consists of computer-generated speech - specifically, the words "left" and "right."
Future consideration of a broader range of coordination tasks that require effective perceptual-motor integration may help to determine the extent that stability and consistency of coordination contributes to the behavioral problems associated with Down syndrome. As well, other ways of presenting perceptual information may serve to optimize perceptual-motor integration. In essence, we are suggesting that efforts to characterize movement coordination in persons with Down syndrome from the perspective of our model of functional cerebral organization, may address a greater range of perceptual-motor behavior that is characteristic of Down syndrome.
Brain-Behavior Relations
Given that much of the previous literature upon which our model is based has employed noninvasive neuropsychological techniques, another direction to be taken in a second round of inquiry is to examine the implications of our model at a level closer to the neural systems themselves. One means of addressing this issue is to adopt an approach fostered by the fields of physiological psychology and cognitive neuroscience and examine the activity of the brain. A basic tenet of the approach is to associate a description of mental events with a description of brain function, along with a characterization of the neural systems that underlie perceptual and motor events and the nature of activity of these systems.
Recent advances in brain imaging techniques have endowed investigators with the tools required to identify and locate active neural systems in the brain during the performance of certain cognitive and motor tasks. Advances in electroencephalography (EEG), as well as the rapid development of magnetoencephalography (MEG), provide non-invasive methods with which to examine cerebral activity. The EEG and MEG, with their high temporal resolution, are particularly suited for the examination of the time-evolving dynamics of cortical activity. The MEG, which measures magnetic fields in the brain, further enhances the spatial resolution compared with that afforded by the EEG. Moreover, progress in analytical methods such as source localization have also enhanced our window into the cortical systems underlying behavior (e.g., see Kristeva et al., 1991; Weinberg et al., 1990). Source localization techniques allow investigators to locate active cortical systems based on EEG potentials and MEG fields recorded at the scalp (e.g., see Wong, 1991). Our model would be considerably strengthened by identifying the neural systems involved during speech perception and movement organization in persons with Down syndrome.
Two important confirmations of our model could result from psycho-physiological studies which a) replicate the behavioral findings of Elliott et al. (1990), and b) examine the pattern of cortical activity associated with cue (visual or verbal) perception and movement preparation. First, our model suggests that the functional systems subserving speech perception in persons with Down syndrome dwell within the right hemisphere. This feature is based upon dichotic listening findings in persons with Down syndrome. Thus, we would expect that during speech perception the underlying cortical systems would be located in the right hemisphere for persons with Down syndrome. Alternatively, our model predicts, and our empirical work suggests, that cerebral organization for movement production in persons with Down syndrome is similar to persons without neurological disabilities (e.g., see Weinberg et al., 1990 for an overview of studies of motor function using the EEG and MEG). Thus, in contrast to what would be predicted by a general "reversal of function" model (cf. Hartley, 1982), we would expect to find that the pattern of cortical activation during movement preparation and execution in individuals with Down syndrome will be similar to that in individuals without neurological disabilities. We are presently preparing this round of inquiry at Simon Fraser University.
Summary
In our work, we have generally applied a neuropsychological, functional systems approach, to the understanding of perceptual-motor integration problems in persons with Down syndrome.
As depicted in Figure 3, the first round of inquiry employed non-invasive neuropsychological techniques to examine the similarities and differences in cerebral organization and perceptual-motor behavior between persons with Down syndrome and chronologically matched control participants. This group differences approach led to the development of a specific model of brain-behavior relations for persons with Down syndrome. The primary feature of the model is the neuroanatomical disconnection of the cerebral areas responsible for speech perception and movement organization. Many of the predictions of the model have been subject to empirical testing and confirmation which, in turn, has allowed use to refine and extend the model. Moreover, the generalizability of the model has been intimated in its promise as a means of predicting individual differences with the Down syndrome population (Elliott and Weeks, 1993). Our present research efforts are directed toward a new round of inquiry that could provide further insight into the specific locus and nature of the brain behavior relations implicated by our behavioral research. In particular, we are extending our investigations to examine other forms of perceptual-motor interactions and to obtain more direct neurophysiological evidence for the model.
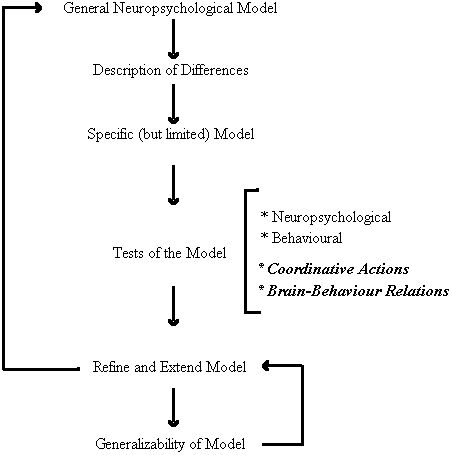
Figure 3. A functional systems approach to the study of perceptual-motor behavior and functional cerebral organization in persons with Down syndrome.
Our long-term goal is to establish guidelines for the development of instructional strategies that may circumvent, or at least reduce, the impact of some of the specific information processing difficulties associated with Down syndrome.
Acknowledgements
The first and second authors gratefully acknowledge the support of the British Columbia Health Research Foundation and the Natural Sciences and Engineering Research Council of Canada. The third author gratefully acknowledges the support of the Ontario Mental Health Foundation.
References
- Ashman, A.F. (1982). Coding, strategic behavior and language performance of institutionalized mentally retarded young adults. American Journal of Mental Deficiency, 86, 627-636.
- Batheja, M. and McManus, I.C. (1985). Handedness in the mentally handicapped. Developmental Medicine and Child Neurology, 27, 63-68.
- Benton, A.L., Varney, N.R., and de Hamsher, K.S. (1978). Lateral differences in tactile direction perception. Neuropsychologia, 16, 109-114.
- Bowler, D.M., Cufflin, J., and Kiernan, C. (1985). Dichotic listening of verbal and nonverbal material by Down syndrome children and children of normal intelligence. Cortex, 21, 637-644.
- Broca, P. (1865). Sur le siege de la faculte du langage articule. Bulletin of the Society of Anthropology, 6, 377-396.
- Bryden, M.P., Bulman-Fleming, M.B., and MacDonald, V. (1996). The measurement of handedness and its relation to neuropsychological issues. In D. Elliott and E.A. Roy, Manual asymmetries in motor performance (pp. 57-81). Boca Raton, FL: CRC Press.
- Byblow, W.D., Chua, R., and Goodman, D. (1995). Asymmetries in coupling dynamics of perception and action. Journal of Motor Behavior, 27, 123-137.
- Carnahan, H. and Elliott, D. (1987). Pedal asymmetry in the reproduction of spatial locations. Cortex, 23, 157-159.
- Cioffi, J. and Kandel, G. L. (1979). Laterality of stereognostic accuracy of children for words, shapes, and bigrams: A sex difference for bigrams. Science, 204, 1432-1434.
- Costa, L.D. (1976). Interest variability on Raven's Coloured Progressive Matrices as an indicator of specific ability deficit in brain-lesioned patients. Cortex, 12, 31-40.
- Denes, F., Semenza, C., and Stoppa, E. (1978). Selective improvement by unilateral brain-damaged patients on Raven Coloured Matrices. Neuropsychologia, 16, 749-752.
- De Renzi, E. (1968). Nonverbal memory and hemispheric side of lesion. Neuropsychologia, 6, 181-189.
- De Renzi, E., Pieczuro, A., and Vignolo, L.A. (1966). Oral apraxia and aphasia. Cortex, 2, 50-73.
- Edwards, J.M. and Elliott, D. (1989). Asymmetries in intermanual transfer of training and motor overflow in adults with Down syndrome and nonhandicapped children. Journal of Clinical and Experimental Neuropsychology, 11, 959-966.
- Elliott, D. (1985). Manual asymmetries in the performance of sequential movement by adolescents and adults with Down syndrome. American Journal of Mental Deficiency, 90, 90-97.
- Elliott, D. and Chua, R. (1996). Manual asymmetries in goal-directed movement. In D. Elliott and E.A. Roy, Manual asymmetries in motor performance (pp.143-158). Boca Raton, FL: CRC Press.
- Elliott, D., Edwards, J.M., Weeks, D.J., Lindley, S., and Carnahan, H. (1987) . Cerebral specialization in young adults with Down syndrome. American Journal of Mental Deficiency, 91, 480-485.
- Elliott, D., Gray, S., and Weeks, D.J. (1991). Verbal cuing and motor skill acquisition for adults with Down syndrome. Adapted Physical Activity Quarterly, 8, 210-220.
- Elliott, D., Pollock, B.J., Chua, R., and Weeks, D.J. (1995). Cerebral specialization in adults with Down syndrome. American Journal on Mental Retardation, 99, 605-615.
- Elliott, D. and Weeks, D.J. (1990). Cerebral specialization and the control of oral and limb movements for individuals with Down syndrome. Journal of Motor Behavior, 22, 6-18.
- Elliott, D. and Weeks, D.J. (1993). Cerebral specialization for speech perception and movement organization in adults with Down syndrome. Cortex, 29, 103-113.
- Elliott, D., Weeks, D.J., and Chua, R. (1994). Anomalous cerebral lateralization and Down syndrome. Brain and Cognition, 26, 191-195.
- Elliott, D., Weeks, D.J., and Elliott, C.L. (1987). Cerebral specialization in individuals with Down syndrome. American Journal on Mental Retardation, 92, 263-271.
- Elliott, D., Weeks, D.J., and Gray, S. (1990). Manual and oral praxis in adults with Down syndrome. Neuropsychologia, 28, 1307-1315.
- Elliott, D., Weeks, D.J., and Jones, R. (1986). Lateral asymmetries in finger-tapping by adolescents and young adults with Down syndrome. American Journal of Mental Deficiency, 90, 472-475.
- Gibson, C., and Bryden, M.P. (1982). Cerebral lateralization in deaf children using a dichaptic task. Paper presented at the annual meeting of the International Neuropsychological Society, Pittsburgh, Pennsylvania.
- Giencke, A. and Lewandowski, L. (1989). Anomalous dominance in Down syndrome young adults. Cortex, 25, 93-102.
- Harris, L.J. (1990). Cultural influences on handedness: Historical and contemporary theory and evidence. In S. Coren, Left-handedness: Behavioral implications and anomalies, pp. 195-258. Amsterdam: North-Holland.
- Hartley, X.Y. (1981). Lateralization of speech stimuli in young Down syndrome children. Cortex, 17, 241-248.
- Hartley, X.Y. (1982). Receptive language processing of Down syndrome children. Journal of Mental Deficiency Research, 26, 263-269.
- Hartley, X.Y. (1986). A summary of recent research into the development of children with Down syndrome. Journal of Mental Deficiency Research, 30, 1-14.
- Heilman, K.M. and Watson, R.T. (1977). The neglect syndrome - a unilateral defect of the orienting response. In S. Harnad, R.W. Doty, L. Goldstein, J. Jaynes, and G. Krauthamer, Lateralization in the nervous system. New York: Academic Press.
- Hicks, R.E. (1974). Asymmetry in bilateral transfer. American Journal of Psychology, 87, 667-674.
- Jackson, J.H. (1958). Selected writings of John Hughling Jackson. J Taylor (Ed.). New York: Basic Books.
- Kelso, J.A.S., DelColle, J.D., and Schoner, G. (1990). Action-perception as a pattern formation process. In M. Jeannerod, Attention and Performance XIII, pp. 139-169. Hillsdale, N.J.: Lawrence Erlbaum.
- Kimura, D. (1966). Dual functional asymmetry of the brain in visual perception. Neuropsychologia, 29, 877-888.
- Kimura, D. (1979). Neuromotor mechanisms in the evolution of human communication. In H.D. Steklis and M.J. Raleigh, Neurobiology of social communication in primates: An evolutionary perspective, pp. 197-219. New York: Academic Press.
- Kinsbourne, M. and Cook, J. (1971). Generalized and lateralized effects of concurrent verbalization on a unimanual skill. Quarterly Journal of Experimental Psychology, 23, 341-345.
- Kinsbourne, M. and Hicks, R.E. (1978). Functional cerebral space: A model for overflow, transfer and interference effects in human performance. In J. Requin, Attention and performance VII, pp. 345-362. New York: Academic Press.
- Kools, J.A., Williams, A.F., Vickers, M.J., and Caell, A. (1971). Oral and limb apraxia in mentally retarded children with deviant articulation. Cortex, 7, 387-400.
- Kristeva, R., Cheyne, D., and Deecke, L. (1991). Neuromagnetic fields accompanying unilateral and bilateral voluntary movements: topography and analysis of cortical sources. Electroencephalography and Clinical Neurophysiology, 81, 284-298.
- Le Clair, D.A. and Elliott, D. (1995). Movement preparation and the costs and benefits associated with advance information for adults with Down syndrome. Adapted Physical Activity Quarterly, 12, 239-249.
- Ley, R.G. and Bryden, M.P. (1979). Hemispheric differences in processing emotions and faces. Brain and Language, 7, 127-138.
- Mahoney, G., Glover, A., and Finger, I. (1981). Relationship between language and sensorimotor development of Down syndrome and nonretarded children. American Journal of Mental Deficiency, 87, 86-95.
- Murphy, M.M. (1962). Hand preferences of three diagnostic groups of severely deficient males. Perceptual and Motor Skills, 14, 508.
- Piccirilli, M., D'Alessandro, P., Mazzi, P., Sciarma, T., and Testa, A. (1991). Cerebral organization for language in Down syndrome patients. Cortex, 27, 41-47.
- Pickergill, M.J. and Pank, C. (1970). Relation of age and Mongolism to lateral preferences in severely subnormal subjects. Nature, 228, 1342-1344.
- Pipe, M.E. (1983). Dichotic-listening performance following auditory discrimination training in Down syndrome and developmentally retarded children. Cortex, 19, 489-491.
- Pipe, M.E. (1988). Atypical laterality and retardation. Psychological Bulletin, 104, 343-349.
- Raven, J.C. (1965). Guide to using the Coloured Progressive Matrices. London: H.K. Lewis.
- Rondal, J. A. (1994). Exceptional cases of language development in mental retardation: The relative autonomy of language as a cognitive system. In H. Tager-Flusberg, Constraints on language acquisition: Studies of atypical children, pp. 155-174. Hillsdale NJ: Erlbaum.
- Roy, E.A. (1985). Neuropsychological studies on apraxia and related disorders. Amsterdam: North-Holland.
- Roy, E.A. and MacKenzie, C.L. (1978). Handedness effects in kinesthetic spatial location judgments. Cortex, 14, 250-258.
- Salmoni, A.W., Schmidt, R.A., and Walter, C.B. (1984). Knowledge of results and motor learning: A review and critical reappraisal. Psychological Bulletin, 95, 355-386.
- Schmidt, R.C., Carello, C, and Turvey, M.T. (1990). Phase transitions and critical fluctuations in the visual coordination of rhythmic movements between people. Journal of Experimental Psychology: Human Perception and Performance, 16, 227-247.
- Semmes, J. (1968). Hemispheric specialization: a possible clue to mechanism. Neuropsychologia, 6, 11-26.
- Smith, S.T., Cash, C., Barr, S.E., and Putney, R.T. (1986). The nonspeech assessment of hemispheric specialization in retarded children. Neuropsychologia, 24, 293-296.
- Tannock, R., Kershner, J.R., and Oliver, J. (1984). Do individuals with Down syndrome possess right hemisphere language dominance? Cortex, 20, 221-231.
- Taylor, H.G. and Heilman, K.M. (1980). Left-hemisphere motor dominance in right-handers. Cortex, 16, 587-603.
- Turvey, M.T. (1990). Coordination. American Psychologist, 45, 938-953.
- Umilta , C., Rizzolatti, G., Marzi, C., Zamboni, G., Franzini, C., Camarda, R., and Berlucchi, G. (1974) . Hemispheric differences in the discrimination of line orientation. Neuropsychologia, 12, 165-174.
- Varga-Khadem, F. (1982). Hemispheric specialization for the processing of tactual stimuli in congenitally deaf and hearing children. Cortex, 18, 277-286.
- Weeks, D.J., Chua, R., Elliott, D., Lyons, J., and Pollock, B.J. (1995). Cerebral specialisation for receptive language in individuals with Down syndrome. Australian Journal of Psychology, 47, 137-140.
- Weeks, D.J. and Elliott, D. (1992). Atypical cerebral dominance in Down syndrome. Bulletin of the Psychonomic Society, 30, 23-25.
- Weinberg, H., Cheyne, D., and Crisp, D. (1990). Electroencephalographic and magnetoencephalographic studies of motor function. In S. Sato, Advances in Neurology 54: Magnetoencephalography, pp. 193-205. New York: Raven Press.
- Wernicke, C. (1874). Der Aphasische Symptomenkomplex. Breslau, Poland: Cohn and Weigert.
- Wimmers, R.H., Beek, P.J., and Van Wierengen, P.C.W. (1992). Phase transitions in rhythmic tracking movements: A case of unilateral coupling. Human Movement Science, 11, 217-226.
- Witelson, S.F. (1974). Hemispheric specialization for linguistic and nonlinguistic tactual perception using a dichotomous stimulation technique. Cortex, 10, 3-17.
- Wong, P.K.H. (1991). Introduction to brain topography. New York: Plenum Press.
- Zekulin-Hartley, X.Y. (1981). Hemispheric asymmetry in Down syndrome children. Canadian Journal of Behavioral Science, 13, 210-217.
- Zekulin-Hartley, X.Y. (1982). Selective attention to dichotic input of retarded children. Cortex, 18, 311-316.

